The GALAH survey examines the chemical changes of the Galaxy over time. Using specific observational techniques, we investigate the different chemical elements in stars of different ages. Not only does this teach us about how elements formed, it also helps us understand the formation of the Milky Way galaxy. Here we summarise some of the astronomical concepts integral to GALAH.
Milky Way Basics
 The Andromeda galaxy. We cannot picture the Milky Way from within it, but astronomers think our own Galaxy looks similar to Andromeda. (Credit: Adam Evans • CC-BY 2.0)
The Andromeda galaxy. We cannot picture the Milky Way from within it, but astronomers think our own Galaxy looks similar to Andromeda. (Credit: Adam Evans • CC-BY 2.0)
The Milky Way galaxy is just one of over 100 billion galaxies in the Universe. Each of these galaxies has over 100 billion stars. The vast quantities of material and structures out there can be a bit intimidating. However, we can use the Milky Way as a Rosetta Stone to understand galaxies in general.
Our Galaxy consists of a relatively flat disk of stars around a bulge of stars in the centre. This is surrounded by a dim, round collection of stars, dubbed the halo. Most of the stars are in the Galactic disk; they have roughly circular orbits in the same direction about the Galactic centre. In contrast, stars in the bulge and halo have much more erratic orbits, varying in height and direction. The Galactic structures also vary in chemistry. Halo stars have very little heavy elements, suggesting that they were some of the first stars to form in the Galaxy, before stellar recycling significantly enriched the environment.
We do not currently understand how these different Milky Way structures formed and evolved. We can use their chemistry and dynamics to examine how the different structures developed. Numerous other galaxies show similar components. Using galactic archaeology, we can investigate the development of the Milky Way, and apply this information to understanding galaxy formation overall.
Element Origins
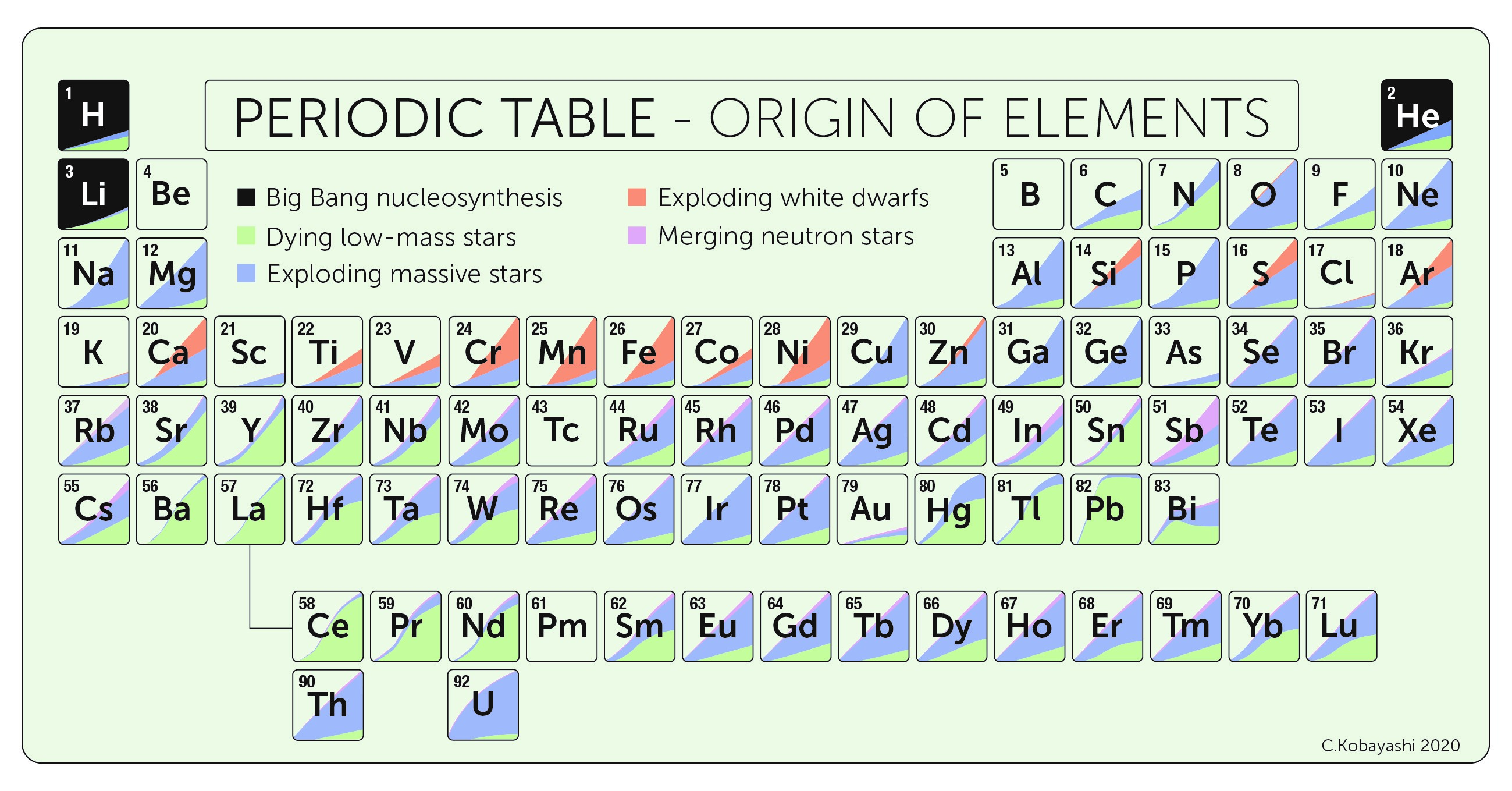 The Periodic Table, showing naturally occurring elements up to uranium. Shading indicates stellar origin. (Content: Chiaki Kobayashi et al.; Artwork: Sahm Keily)
The Periodic Table, showing naturally occurring elements up to uranium. Shading indicates stellar origin. (Content: Chiaki Kobayashi et al.; Artwork: Sahm Keily)
After the Big Bang, the ordinary matter in the Universe was composed primarily of just two elements: hydrogen and helium. Currently, there are a wide range of elements, from argon to zirconium. These more complex elements were nearly all produced via stellar evolution processes. The formation of new elements is called nucleosynthesis. Below, we describe the basics of the chemistry of the Universe and how it has changed over time.
Big Bang Nucleosynthesis
When the Big Bang occurred approximately 14 billion years ago, the Universe was very hot and dense. Over time, the Universe expanded and cooled down. Approximately 0.001 seconds after the Big Bang, particles (e.g., protons and neutrons) began to fuse together to form atomic nuclei, dubbed nuclear fusion. Nuclear fusion processes depend on the density and temperature of the environment. More complex atomic nuclei require dense, hot environments; particles are more likely to smack into each other and stick together in these conditions. Although the Big Bang gave rise to an incredibly dense and hot environment, it was also expanding at a great rate. Approximately 5 minutes after the Big Bang, the Universe was no longer hot and dense enough to continue fusion. At this point, 75% of the ordinary matter in the universe (by mass) was Hydrogen nuclei. 25% had combined into helium nuclei. There were also trace amounts of other elements, namely deuterium (a hydrogen atom with an additional neutron) and lithium. This process is dubbed Big Bang Nucleosynthesis (BBN).
The chemical make up of the Universe is still representative of BBN. However, it also has a small fraction of more complex elements, which astronomers refer to as metals or heavy elements, produced via stars.
Stellar Nucleosynthesis
 The star-forming region 30 Doradus. The blue stars are massive, hot, young stars. They have formed in a cluster out of gas and dust. (Credit: NASA, ESA, F. Paresce, R. O’Connell, and the WFC3 Science Oversight Committee)
The star-forming region 30 Doradus. The blue stars are massive, hot, young stars. They have formed in a cluster out of gas and dust. (Credit: NASA, ESA, F. Paresce, R. O’Connell, and the WFC3 Science Oversight Committee)
Over time, the products of BBN gravitationally coalesced into clumps of material which collapsed to form the first stars. As this material collapsed, the temperature and density of the central matter increased. Eventually, the central pressure balanced with the inward pull of gravity. Similar to BBN, the hot, dense stellar core contained energized particles, primarily hydrogen atoms, zooming around. When some of these particles collided, they combined to form more complex elements. This fusion released energy as radiation, which supported the core from gravity and, over a period of time, escaped to space from the less-dense stellar atmosphere.
A star’s lifetime depends both on its mass, which reflects the amount of available fuel for nuclear fusion, and its luminosity, the rate at which it uses up this fuel and releases radiation to space. These first stars were very massive, with vast quantities of fuel. However, they were incredibly luminous and lived for only a few million years. When these stars ran out of fuel, they released their stellar material into the surrounding environments, including the more complex elements produced through nuclear fusion in the stellar core.
The next generation of stars formed out of material that had been enriched by the nuclear fusion products of the first. Just like the first generation, these stars initially burn hydrogen in their cores, fusing it into helium atoms. After a period of time, the star has processed much of the core hydrogen and this fusion process no longer releases enough energy to counteract the inward pull of gravity. The stellar core contracts, reaching temperatures and densities that support nuclear fusion of helium into carbon. Hydrogen fusion persists in less dense portions of the star. For more massive stars, this pattern continues as the star creates more elements, such as carbon, neon, oxygen, silicon, and iron. Whereas for elements less massive than iron, nuclear fusion released energy, beyond this element fusion requires an energy source. Once the material in a stellar core has fused to iron, it can no longer produce energy and support itself from gravitational collapse. The star will collapse and explode as a supernova, releasing all of the elements it produced through fusion into its surroundings. There are additional nucleosynthetic processes that occur in supernovae and, via neutron-capture, form elements heavier than iron, such as europium.
Low-mass stars, like the Sun, have a simpler life than those with high mass. The lowest mass stars are unable to reach appropriate temperatures to fuse helium, ending their lives as white dwarfs. For those that can, core nuclear fusion cannot move beyond carbon. It takes very large temperatures, which low mass stars are unable to reach, to fuse carbon. Without pressure support, the core gravitationally shrinks. This increases the temperature and density of material that surrounds the core, igniting hydrogen and helium fusion in layers. Fusion in these shells occurs at a much more rapid rate than in the core, producing large amounts of energy. This results in the shells expanding outwards, as the pressure support overcomes the gravitational pull. Astronomers suspect that there are additional nucleosynthetic processes that occur at this life stage; through neutron-capture, the stellar material combines to form elements beyond iron. Stellar winds then remove material from these puffed-up outer layers, releasing the enriched material into the surrounding environment. Eventually, these outer layers are ejected into space from the carbon stellar core, creating what astronomers call a planetary nebula.
Stars take hydrogen and helium from BBN, and through various nuclear fusion processes, form other elements, namely, carbon, oxygen, silicon, sulfur, and iron. When these stars can no longer support themselves via fusion processes, their deaths release their processed material into their surrounding environment. The next generation of stars forms out of this enriched material and, upon its death, releases the complex elements it produced. Over time, the chemistry of the Universe gets increasingly complex and varied thanks to this cycle of stellar recycling. After BBN, approximately 99.9% of material was hydrogen and helium. Now, after over 10 billion years of recyling, approximately 2% of material is made of more complex elements. That may not seem like much, but consider that everything around you was produced during a star’s life and death!
Galactic Archaeology
Stellar recycling gradually contributes heavy elements to the Universe. When a star forms, its chemistry reflects that of the material it formed from. For example, a star formed very early on in the Galaxy will have a small amount of heavy elements. Stars formed more recently will have a greater proportion of metals. In the same way that fossilized plants and animals tell us about early conditions of the Earth, we can use stars as a “fossil record” of the chemical evolution of the Milky Way. In addition, by identifying stars with common origins via their chemical signature, we can understand the formation and development of the Milky Way galaxy. This technique is called Galactic archaeology.
Stars form in clusters from a parent cloud of gas and dust. Each star formed in this cluster at a given time should have a similar chemical signature. Due to stellar motion in the Galaxy, stars that formed together are not necessarily still together. Fortunately, the chemical abundances in an individual star are like stellar DNA; they tell us about where and when the star formed. Elemental abundances allow us to track down stellar “siblings,” that are now spread throughout the Milky Way. Using the abundance distribution of stars to recognise relic stars from old long-dispersed star formation sites is called chemical tagging.
Chemical abundances can also identify stars that were not formed in the Galaxy. Over the course of its long lifetime, the Milky Way encounters other galaxies. Oftentimes, it will gravitationally strip gas and stars from these. Using chemistry, we can identify stars that were cannibalized from other stellar bodies.
Examining the elements in a star teaches us about the kinds of stars that were in the preceding generation. For example, if a star has a large proportion of elements formed in supernova, that suggests it formed from material enriched by an massive star that died in a supernova. In turn, this can also help us better understand the different stellar nucleosynthetic processes and in which kinds of stars they occur.
Spectroscopy
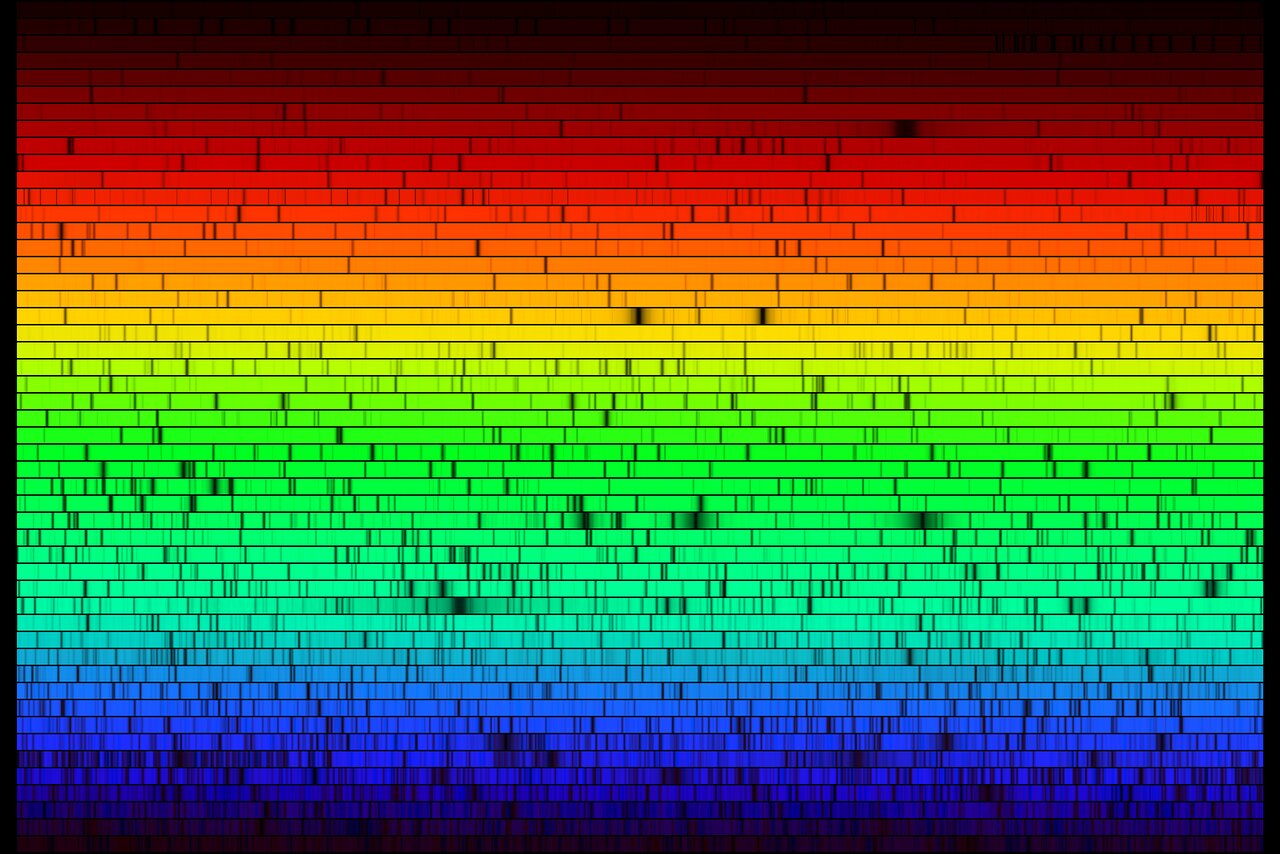 A high resolution version of the spectrum of our Sun. (Credit: N.A.Sharp, NOAO/NSO/Kitt Peak FTS/AURA/NSF)
A high resolution version of the spectrum of our Sun. (Credit: N.A.Sharp, NOAO/NSO/Kitt Peak FTS/AURA/NSF)
To figure out the different elements, and their proportions, in stars we use spectroscopy. Spectroscopy is an observational technique that separates light by its energy. For example, when you pass light through a prism, you see a rainbow of colors. The prism is separating out the white light by energy, with blue being the most energetic and red the least. The energy of light is referred to as the wavelength; the larger the wavelength, the lower the energy. For example, infrared light is lower energy, with wavelengths greater than 8000 Å. Ultraviolet light is higher energy, with wavelengths less than 4000 Å.
Different elements absorb and emit different kinds of light based on the structure of their nucleus. Each atom, ion, and molecule has a unique set of energy levels. An electrons can only move from one specific energy level to another in the atom. When electrons move up an energy level, they absorb availble light. When they move down, they release it. This results in each individual atom, ion, and molecule being associated with light of specific energies. By looking at the different kinds of light that come from a star, we can determine what elements are present and in what amounts.
When GALAH observes a particular star, we take the light and break it up by wavelength. When a particular element is in the stellar atmosphere, it will absorb certain wavelengths of light. By looking at where we are missing light from a spectrum, we can identify the different elements present. When there is a large amount of a particular atom, ion, or molecule in the stellar atmosphere, its spectral feature is typically stronger. Using line strength, in comparison of models of stellar atmospheres, we can determine the different elemental abundances for individual stars.
This video shows how light from objects in the sky passes through the telescope and is split into a spectrum for scientists to analyse. Credit: Dr. Amanda Bauer (Australian Astronomical Observatory)